Abstract
Introduction: Pegylated interferon alfa-2a (PEG-INF-alfa-2a) is capable of inducing high hematologic (HR) and even complete molecular responses (MR) in patients with polycythemia vera (PV) and essential thrombocythemia (ET). We have recently reported that about half of these responses are not durable. Biomarkers to predict response to treatment or its durability are unknown.
Objective: We analyzed molecular mutations (mut.) in ET/PV patients treated with PEG-INF-alfa-2a to potentially identify predictive markers associated with therapeutic response.
Methods: Targeted next generation DNA sequencing was performed on paired samples (bone marrow or peripheral blood) obtained at study entry and during therapy. The entire coding regions of 14 recurrently mutated genes and 35 hotspot mutations harboring exons in patients with myeloid malignancies were sequenced using the Illumina MiSeq platform, and analyzed with NextGene Software. Gene mutation findings were correlated with clinical characteristics using the Fisher's exact, or Mann-Whitney U tests, as appropriate. Hematologic (HR) and molecular response (MR) assessments were as previously reported (Masarova, Blood, 2015).
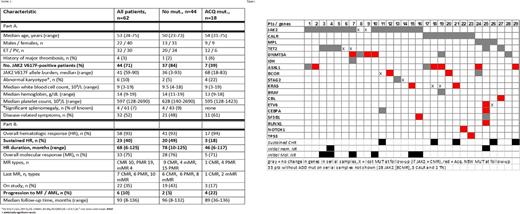
Results: Paired samples of 62 patients (32 ET, 30 PV) were analyzed. Median time between samples was 71 months (range, 7-106), and median treatment duration was 82 months (range, 10-136+). Median disease duration before study enrollment was 37 months (range, 0.1-360), with 40% of patients being diagnosed for > 5 years. Forty-three (67%) patients were previously treated. After a median follow-up of 94 months (range, 8-136+), 22 (35%) patients are on study. Clinical and demographics characteristics of all patients are shown in Table 1. Forty-four (71%) patients carried JAK2 V617F, 10 (16%) CALR, 3 (5%) MPL mut., and 5 were triple negative (TN, 8%). Overall, 93% had an initial HR and 75% a MR (JAK2+ only), and these responses were sustained in 40% and 30% of patients, respectively.
Thirty-four (55%) patients had 0-1, 19 (31%) had 2, and 9 (15%) ³ 3 mut. Except for higher platelets in patients with ³3 mut., and more ET patients in this group (8 ET, 1 PV, 25% vs 3%, p=0.014), clinical characteristics were similar among groups. Thirty-three patients (53%) had no additional mut. (ADD mut., except JAK2, MPL and CALR); 21 had one, 4 had two, and 4 had three or more. Twenty-nine percent of patients had ADD mut. before treatment (higher frequency in ET patients, 34 vs 23% in ET vs PV, p=0.04), 42% during treatment, and 24% in both samples. Frequency, type of mut., and changes over time are detailed in Figure 1.
Eighteen patients (29%) acquired a new mut. (ACQ mut.) during therapy, 3 of whom are still being actively treated. JAK2 mut. was less frequent in patients with ACQ mut. (39 vs 84%, p=0.002); no other clinical differences were observed (Table 1). Likewise, more patients with CALR and MPL mutations ACQ mut. (16% JAK2, 70% CALR, 67% MPL and 40% TN, p=0.002). Overall, JAK2- patients had 3.5-fold higher odds of ACQ mut. than JAK2+ patients (p=0.02, odds 3.5, 95% CI 1.23-10.1). There was no difference in the frequency of ACQ mut. between ET and PV patients (p>0.05).
The presence of initial ADD mut, their number, or type had no impact on durability of initial HR. Acquisition of mut. directly correlated with loss of initial HR; patients with ACQ mut. had a 3.8-fold higher chance of losing their HR than those without (78 vs 48%, p=0.04, odds 3.8, 95% CI 1.09-13.5). Similarly, patients with ACQ mut. had a shorter duration of HR (53 vs 89 months, p = 0.02). Six patients progressed to myelofibrosis (n=5) or acute leukemia (n=1) while on therapy, with a higher frequency among patients with ACQ mut. (22% vs 4%, p=0.03).
Among JAK2+ patients, the presence, number of ADD mut. and ACQ mut. were not correlated with MR, its type or durability. Among 10 patients with initial ADD mut., 2 had no MR, 7 had a partial MR (PMR) and 1 had a complete MR (CMR). Among 7 patients who ACQ mut., 2 had no MR, 4 had a PMR and 1 had a CMR. The frequency of ACQ mut. was similar between patients with and without a sustained MR (9% vs 18%, p>0.05).
Conclusions: The acquisition of mutations, as evidence of clonal evolution, is seen in ET and PV patients treated with PEG-INF-alfa-2a, and correlates with a shorter duration of clinical benefit, and a shorter duration of hematologic response. Among JAK2+ patients, no association between the acquisition of new mutations and molecular response was found.
Cortes: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Sun Pharma: Research Funding; ImmunoGen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Teva: Research Funding; Pfizer: Consultancy, Research Funding. Kantarjian: Novartis: Research Funding; Delta-Fly Pharma: Research Funding; Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding. Verstovsek: NS Pharma: Research Funding; Galena BioPharma: Research Funding; Gilead: Research Funding; Galena BioPharma: Research Funding; Bristol Myers Squibb: Research Funding; Blueprint Medicines Corp: Research Funding; Pfizer: Research Funding; CTI BioPharma Corp: Research Funding; Lilly Oncology: Research Funding; Celgene: Research Funding; Celgene: Research Funding; Roche: Research Funding; Lilly Oncology: Research Funding; Pfizer: Research Funding; CTI BioPharma Corp: Research Funding; NS Pharma: Research Funding; Genentech: Research Funding; Promedior: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Roche: Research Funding; Astrazeneca: Research Funding; Gilead: Research Funding; Blueprint Medicines Corp: Research Funding; Incyte: Research Funding; Genentech: Research Funding; Promedior: Research Funding; Bristol Myers Squibb: Research Funding; Astrazeneca: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal